Blog & News
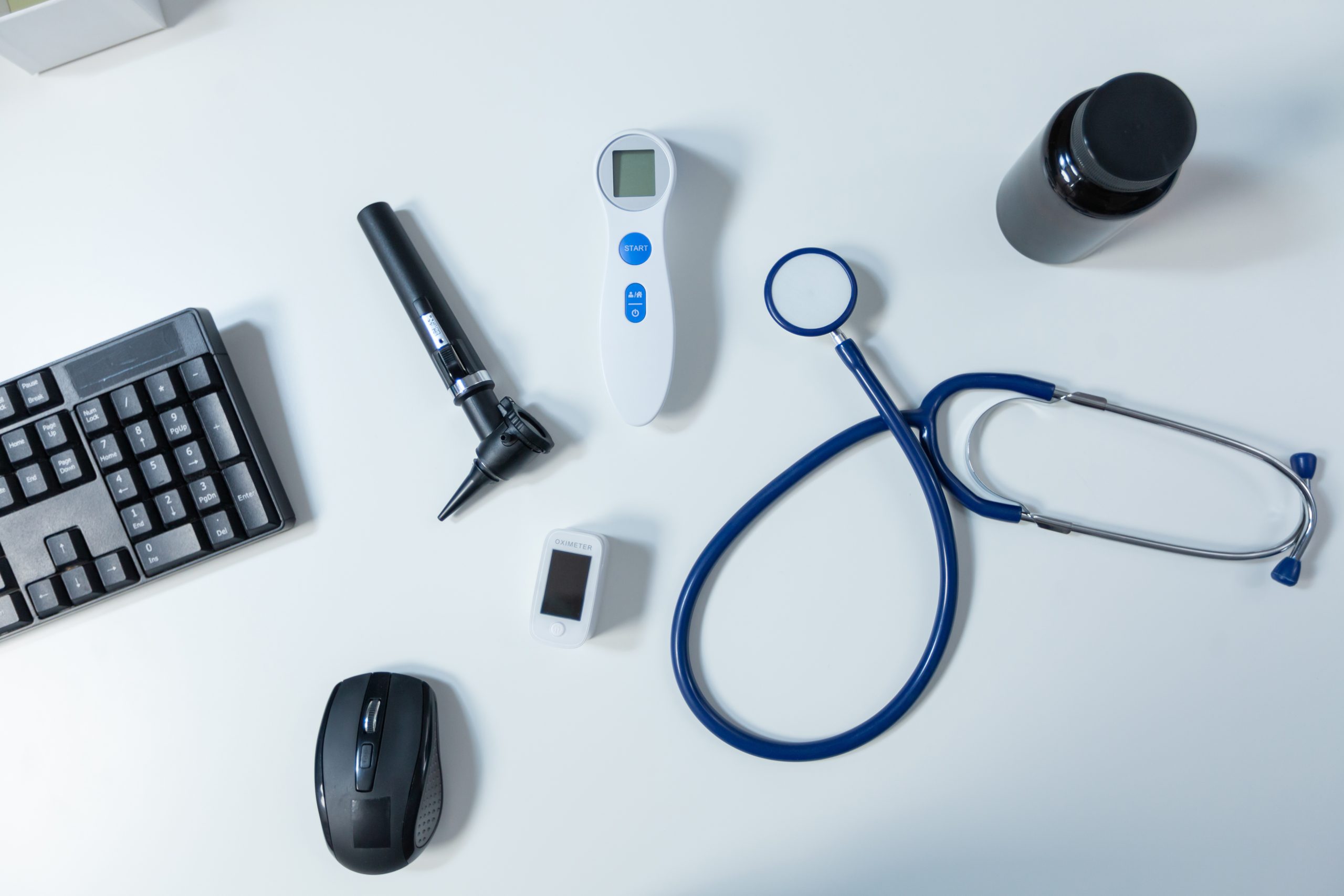
Medical Device Classifications: Which Risk Group Is Your Product In?
Classification Is the Starting Point for CE Certification If you want to sell a medical device in the European Union, the first and most crucial

How to Build Quality Control Systems in Medical Textile Machinery
Quality Is Not a Coincidence—It’s a System In medical textile production, your products interact directly with patient health. That means quality control isn’t just about

What’s the Difference Between MDR and ISO 13485?
Both Are Essential — But Not the Same If you’re in the medical manufacturing field, you’ve probably heard questions like “Are you MDR-compliant?” or “Do

How to Obtain CE Certification in Medical Manufacturing
What Is CE Certification and Why Does It Matter? The CE mark (Conformité Européenne) indicates that a product complies with EU regulations and can be

Essential Certifications for Medical Device Manufacturing: CE, MDR, ISO 13485 & More
Why Certifications Matter in Medical Production In the medical device industry, it’s not enough to produce high-quality goods. To sell your products—especially in regulated markets

Why Preventive Maintenance Is Key to Continuous Production
The Cost of Downtime in Medical Production In medical textile manufacturing, every minute of downtime is costly—not just in terms of lost output, but also

